This blog collects great science and math work made by students from around the world. All videos and other content are added by science teachers. The blog is open to anyone who like to share great work for others to see and gain from.
Contact Michael Ljunggreen by mail: mich0771@skole.kolding.dk
11/12/11
Forces of Air
7th. grade made this experiment with a can and a tub of water. Try making the same experiment (but be careful).
11/10/11
Water and Air
This experiment is made by 7. grade at Vonsild School. What do you think is causing the result?
Dette eksperiment er lavet af 7. klasse på Vonsild Skole. Hvad tror du er årsagen til resultatet af forsøget?
Location:
Vonsild Skole, 6000 Kolding, Danmark
11/7/11
Densitet i faste stoffer
Gruppe 1: Mads Frost, Magnus Schou og Mikkel Andersen.
Et fast stof tager ikke form efter den beholder den anbringes i. Når et fast stof bliver opvarmet ville det vokse, de fleste faste stoffer ville øge deres størelse med 0,02 procent når temperaturen stiger 10 grader celsius. Så hvis et fast bliver opvarmet med 100 grader ville det vokser 2 cm. Densitet betder ''gram pr. kubikcentimeter. Det eneste stof som har densitet på præcis 1 gram pr. kubikcentimeter. Når et stof opvarmes stiger densiteten. Det vil sige at moleklerne i stoffet bevæger sig hurtigere og derfor skal bruge mere plads. Derfor udvides et stof når det varmes op. Det stof der har den højeste densitet er Stoffet Osmium som bruges til fremstilling af mikroskoper og ling.
Onsdag d. 7 September lavede vi et forsøg med Densitet af faste stoffer som F.eks. Træ, Jern og guld. For at måle densiteten af et fast stof skal man have et måleglas eller lignende. Vi bugte et glas på 100 ml. Og så fylder man vand op til de 50 ml. Derefter ved vi at densiteten af vand er præcis 1g/kubikcentimeter, så for at aflære hvad densiteten er kan man bare så på hvor meget vandet stiger. Ved F.eks. Guld stiger vandet 19,3 milliliter, det vil alså sige at vi har en densitet af guld på 19,3g/kubikcentimeter. Vi har samlet et par billeder til jer omkring emnet:
Fårmålet med forsøget er at man skal kunne vide hvilket stof man har at gøre med. F.eks. idag hvis man nu finder noget guld. Kan man vide præcis at det er guld ved at finde densiteten af stoffet.
På billeder 1: vises hvad densitet er og hvordan man kan finde ud af resultatet. På billede 2: Vises der hvordan man måler densitet af et fast stof og Densiteten der er 12,4 gram pr. kubikcentimeter. Og det er stoffet sølv. I videoen kan du se hvad der sker når moleklerne er hurtigere og stoffet udvides.
Et fast stof tager ikke form efter den beholder den anbringes i. Når et fast stof bliver opvarmet ville det vokse, de fleste faste stoffer ville øge deres størelse med 0,02 procent når temperaturen stiger 10 grader celsius. Så hvis et fast bliver opvarmet med 100 grader ville det vokser 2 cm. Densitet betder ''gram pr. kubikcentimeter. Det eneste stof som har densitet på præcis 1 gram pr. kubikcentimeter. Når et stof opvarmes stiger densiteten. Det vil sige at moleklerne i stoffet bevæger sig hurtigere og derfor skal bruge mere plads. Derfor udvides et stof når det varmes op. Det stof der har den højeste densitet er Stoffet Osmium som bruges til fremstilling af mikroskoper og ling.
Onsdag d. 7 September lavede vi et forsøg med Densitet af faste stoffer som F.eks. Træ, Jern og guld. For at måle densiteten af et fast stof skal man have et måleglas eller lignende. Vi bugte et glas på 100 ml. Og så fylder man vand op til de 50 ml. Derefter ved vi at densiteten af vand er præcis 1g/kubikcentimeter, så for at aflære hvad densiteten er kan man bare så på hvor meget vandet stiger. Ved F.eks. Guld stiger vandet 19,3 milliliter, det vil alså sige at vi har en densitet af guld på 19,3g/kubikcentimeter. Vi har samlet et par billeder til jer omkring emnet:
Fårmålet med forsøget er at man skal kunne vide hvilket stof man har at gøre med. F.eks. idag hvis man nu finder noget guld. Kan man vide præcis at det er guld ved at finde densiteten af stoffet.
På billeder 1: vises hvad densitet er og hvordan man kan finde ud af resultatet. På billede 2: Vises der hvordan man måler densitet af et fast stof og Densiteten der er 12,4 gram pr. kubikcentimeter. Og det er stoffet sølv. I videoen kan du se hvad der sker når moleklerne er hurtigere og stoffet udvides.
Labels:
Dansk
Location:
Vonsild Skole, 6000 Kolding, Danmark
Væsker i lag
1. Varmt vand er lettere end koldt.
Prøv eventuelt dette forsøg.
Du skal bruge to cylinderglas, salt, frugtfarve og madpapir.
1. Hæld tre spiseskefulde salt i et cylinderglas.
2. Fyld op med vand, og rør rundt til saltet er opløst. Hæld derefter mere vand i til glasset er fyldt.
3. Hæld vand i et andet glas og tilsæt frugtfarve hæld derefter vand i glasset til det er fyldt, læg et stykke madpapir over.
4. Vend glasset med madpapir på hovedet og sæt det omvendt på glasset med saltvand.
5. Træk forsigtigt papiret mellem de to glas væk.
6. Tegn opstillingen.
7. Vend glassene om så glasset med frugtfarve står øverst.
8. Hvad sker der (hvis i laver forsøget så skriv venlist hvad der sker i en kommentar)
Prøv eventuelt dette forsøg.
Du skal bruge to cylinderglas, salt, frugtfarve og madpapir.
1. Hæld tre spiseskefulde salt i et cylinderglas.
2. Fyld op med vand, og rør rundt til saltet er opløst. Hæld derefter mere vand i til glasset er fyldt.
3. Hæld vand i et andet glas og tilsæt frugtfarve hæld derefter vand i glasset til det er fyldt, læg et stykke madpapir over.
4. Vend glasset med madpapir på hovedet og sæt det omvendt på glasset med saltvand.
5. Træk forsigtigt papiret mellem de to glas væk.
6. Tegn opstillingen.
7. Vend glassene om så glasset med frugtfarve står øverst.
8. Hvad sker der (hvis i laver forsøget så skriv venlist hvad der sker i en kommentar)
Labels:
Dansk
Location:
Vonsild Skole, 6000 Kolding, Danmark
Tilstandsformer

Fast form:
Et fast stof bevarer altid sin form. Hvis et fast stof anbringes i en beholder, tager den ikke form efter beholderen. I et fast stof bevæger molokylerne sig ikke, men stiger temperaturen begynder molokylerne så småt at bevæge sig hurtigere. På en frostdag hvor temperaturen er under nulpunktet, har de en gennemsnitsfart på 440 meter pr. sekund. Når molokylerne
overhovedet ikke bevæger sig, kan temperaturen slet ikke blive koldere og det absolutte nulpunkt er -273 grader. Hvis man varmer et fast stof op, vil det normalt udvide sig lidt. Flere faste stoffer udvider sig omkring 0,02 procent, når temperaturen stiger ca. 10 grader.
Væske:
En væske som hældes i en beholder, vil ligge sig på bunden af beholderen og væsken vil have en vandret overflade. En væske kan være tyktflydende ligesom ketchup og sirup. Væsker udvider sig normalt ved opvarmning. Som en væske kan længden ikke ændre sig. Udvidelsen beskrives nærmest som en ændring i rumfanget.
Luft form:
Når et stof er en luft form, vil det udfylde beholderen helt, hvis du putter den i en beholder. En luft art ses normalt ikke. Luften, vi indånder, har også en densitet den er bare meget lille. Det luft vi indånder kan også blive til et fast stof og en væske. (Lidt om gasarter). Det luft vi indånder er oxygen, kvælstof og lidt CO2. Luft er en gasart og CO2 er begge gasarter. Methangas er en blanding af et kul atom og fire brint atomer, det er den naturgas vi varmer vores hus op med idag.
Luft form:
Når et stof er en luft form, vil det udfylde beholderen helt, hvis du putter den i en beholder. En luft art ses normalt ikke. Luften, vi indånder, har også en densitet den er bare meget lille. Det luft vi indånder kan også blive til et fast stof og en væske. (Lidt om gasarter). Det luft vi indånder er oxygen, kvælstof og lidt CO2. Luft er en gasart og CO2 er begge gasarter. Methangas er en blanding af et kul atom og fire brint atomer, det er den naturgas vi varmer vores hus op med idag.
Hvorfor er det vigtigt at vide noget om disse tilstandsformer? Det er vigtigt at vide noget om et fast stof fordi: (Eksempel) Hvis du gerne vil have en drink med et par isterninger i, så er det nok også en god idé og kunne kende frysepunktet, som er -273, så du kan fryse væsken ned som dannes til et fast stof! Det er også vigtigt at vide noget om en væske fordi: (Eksempel) Væske er en livsvigtig ting og det fylder det meste af verden, så hvis der ingen væske var, så var der heller ingen menneskehed! Det er også vigtigt at vide noget om en luft form fordi: (Eksempel)
Du vil gerne vide hvordan at du kan lave te, får at vide det skal du have damp, men er damp ikke varmt hvis det kommer i en bestemt temperatur? Jo, så det vigtige er der at du jo ikke skal hælde dampende vand ud over dig!
Lavet af Caroline og Michella (7.B)
Labels:
Dansk
Location:
Vonsild Skole, 6000 Kolding, Danmark
Nuclear physics and radiation in the weekday
There are 3 types of radiation. Alpha, Gamma and Beta.
Alpha-radiation:
Alpha-radiation consists of two neutrons and two protons.That’s the Helium element. Alpha is the biggest radiation of Beta and Gamma radiation. Alpha is a very powerful radiation, that can damage your body, because it is so massive. Alpha has a speed of 15.000 km/s and that’s 5% the speed of light. But Alpha looses its energy really fast. That’s why it has a short range. Alpha can’t penetrate your skin and go through your body. And that’s why it is so dangerous. With the speed of light and that it can’t penetrate your skin, does that it has a powerful force, which will hit your skin with a massive amount of burst. If you inhale materials with a high amount of Alpha it can really damage your body.
Beta-radiation:
Beta-radiation has a longer range, and it is more penetrating. Beta emissions one electron or a positron. A positron is like an anti-electron. If a positron ever will come in contact with an electron it will be an annihilation. Because in the annihilation, 100% of the mass is converted into energy. Beta-radiation is not so dangerous as Alpha. If Beta radiation hits your body it will begin to damage it. It takes long time before you can feel/see that Beta has damaged your body, because it takes longer time for Beta to hurt your body than Alpha does. Worst of all you can get cancer. Young children especially new born children are vulnerable.The energy Beta sends out, is far more than Alpha, but less than Gamma. Like Alpha, Beta travels at the speed of light.
Gamma-radiation:
Gamma is unlike the other radiations. While the two others have an element or a mass, Gamma is just pure energy. Electromagnetic energy (EM). It takes a paper to stop Alpha. it takes an iron plate to stop Beta. But with Gamma it takes more than that. It takes a block of lead. Because as you know Alpha has a high mass, because it consists of the Helium element. That’s why it only takes a paper to stop it. Beta has a small particle. It’s not near as big as Alpha because it needs an iron plate to stop it. Because Beta sends out an electron or a positron. An electron is 2000 times smaller than a proton. Remember a proton and a positron is not the same! Gamma is energy, and that’s why it takes more than an iron plate to stop it. So why can lead stop it? As it is, Gamma belongs in the EM wave class. That means that EM waves get absorbed during the penetration of the lead. What absorbs the energy? It’s the electrons. They absorb the energy and converts it into heat or light. Because Gamma can generate heat with the help of the electrons from the lead, it can maybe in the future be used in many products. But the main source of this, will be to use it to make electricity. You could also combine positrons and a electrons in a controlled environment. That will force them together and make an explosion and form energy, used to make electricity.
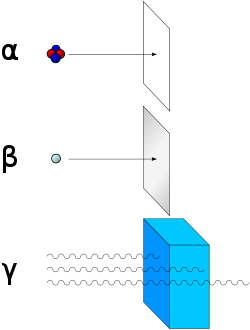
Alpha-radiation is not very penetrating, so that means that a little simple paper can stop the radiation. There need to be 8 pieces of paper to stop Alpha-radiation.
Beta-radiation is a little bit more penetrating than Alpha, so Beta can’t be stop by paper. Aluminium can stop Beta-radiation. There need to be 8 pieces of paper and round about 2 mm aluminium.
Gamma-radiation is very penetrating, so it is hard to stop the energy. There need to be 7 cm lead before Gamma is slowed down.
Everyone on the earth, is exposed to radiation every hour. But it isn’t dangerous. Our organism is build for that, so it can’t hurt us. We can’t do anything to stop the daily radiation. This is called background radiation.
What do we use Alpha-radiation for?
Alpha is used in fire alarms or smoke detectors because in the fire alarm there is some Americium-241. There is like 1/5000 of a gram in it, but Americium sends out Alpha radiation. Americium constantly releases Alpha which knocks the electrons of the air. It ionizes the oxygen and nitrogen which are in the chamber. The positive oxygen and nitrogen are attached to the negative plate while the electrons are attached to the positive plate. That generates a small electric current. When smoke enters the chamber, the smoke particles attach to the ions and neutralise them. The effect of that is, that the ions don’t reach the plate. It stops the electric current and triggers the fire alarm.The Alpha particle is also in the atomic bomb. Uranium-235 sends out Alpha particle. The Alpha particle hits the other Uranium atoms and a nuclear fission is about to be created instantly. When a fission happens it generates a lot of heat, and then the big iron barrel can’t hold the pressure anymore.
What do we use Beta-radiation for?
We use Beta-radiation to cure people of cancer. When people get cancer in their cells, they go to a hospital. At the hospital they get radiotherapy. A machine, shoot radiation beams, at the place where you have got cancer. The radiation beams destroy the cells in which you have got cancer, and that will help you out of cancer. There are some side effects. When the machine shoot radiation beams at the place in which you have got cancer, you also loose the good cells. The worst that can happen is that you can loose your hair or you can get sterile. You can only be sterile if you get radiation beams in your testicles.
What do we use Gamma-radiation for?
Often when a fusion happens it creates energy in form of Gamma radiation. Gamma rays are the most energetic form of light and are produced in the very hot area of the universe. They are also produced in a supernova explosion. A supernova is a way to describe how massive stars die, like you have heard before, our sun is a star, not a planet. When the sun gets older and older, it grows to a massive size. By the end, the size of the sun will fill the whole solar-system and completely destroy all the planets. When the sun is at it’s biggest, it explodes. Not just a normal tiny explosion, but an explosion that is so powerful that it could erase the Milkyway. That is a supernova. Gamma rays are used in astronomy. Every star, supernova, black hole, solar flares and active galaxies send out gamma rays. When we invented the telescope which could see gamma rays, everything became much more clear. We could now experience a whole new level of exploring the galaxies.
Nuclear Phychics
Nuclear phychics were discovered in 1896 by a man called Henri Becquerel. In a coincidence he discovered that uranium salt blackened his photographic plates. He let the uranium salt be in his window for about 3 hours. The uranium salt was put on top of photographic plates. The uranium salt was wrapped in photographic film, so the sunshine couldn’t shine at the uranium salt. That is how he discovered it. In 1903 he got the Nobelprice for that discovery. Later they discovered that the reason of the blackened photographic plates was the changes in the unstable nuclear core.
This is also about radiation. If something emits Alpha radiation, it gives out 2 protons and 2 neutrons. Alpha is the largest radiation beam which exists. So it will go from one element to another.This is the solution:241Am237Np+4He. If you got 241Am and it releases alpha radiation, then that means 4helium molecule, travel away from it. Then it gets 4 less nucleons and then it becomes another element 237Np. But of course it takes time before an element becomes another element. That is what we call half-life. Half life is the time it takes to become another element. When a half life is gone 50% of it is covered into another element, now in this reason it’s Neptunium. When another half life is gone, 50% percentages of the rest of the Americium is covered into Neptunium. So now there’s only 25% left. This will happen until all of it, is covered into Neptunium. One half life of Americium is 432 years.
This doesn't happen in Beta radiation. In Beta radiation something else happen. Beta radiation sends out one proton and one electron. This happens when a neutron is transmuted into a proton and an electron. When a core gets another proton it becomes another element.
So Strontium sends out an electron and a proton. That will then say that the next element will get another proton. In this case it’s another Strontium molecule. When that happens it get another proton and therefore it will change into another element. The half life of Strontium is right around 29 years.
Gamma radiation is a electromagnetic beam. It is pure energy, and when an element emits gamma, none element transfusion happens. It doesn’t change into another element, it stays the same. Gamma radiation is in the same league as X-rays and Radio waves.
By Emil and Mikkel (9.A)
Alpha-radiation:
Alpha-radiation consists of two neutrons and two protons.That’s the Helium element. Alpha is the biggest radiation of Beta and Gamma radiation. Alpha is a very powerful radiation, that can damage your body, because it is so massive. Alpha has a speed of 15.000 km/s and that’s 5% the speed of light. But Alpha looses its energy really fast. That’s why it has a short range. Alpha can’t penetrate your skin and go through your body. And that’s why it is so dangerous. With the speed of light and that it can’t penetrate your skin, does that it has a powerful force, which will hit your skin with a massive amount of burst. If you inhale materials with a high amount of Alpha it can really damage your body.
Beta-radiation:
Beta-radiation has a longer range, and it is more penetrating. Beta emissions one electron or a positron. A positron is like an anti-electron. If a positron ever will come in contact with an electron it will be an annihilation. Because in the annihilation, 100% of the mass is converted into energy. Beta-radiation is not so dangerous as Alpha. If Beta radiation hits your body it will begin to damage it. It takes long time before you can feel/see that Beta has damaged your body, because it takes longer time for Beta to hurt your body than Alpha does. Worst of all you can get cancer. Young children especially new born children are vulnerable.The energy Beta sends out, is far more than Alpha, but less than Gamma. Like Alpha, Beta travels at the speed of light.
Gamma-radiation:
Gamma is unlike the other radiations. While the two others have an element or a mass, Gamma is just pure energy. Electromagnetic energy (EM). It takes a paper to stop Alpha. it takes an iron plate to stop Beta. But with Gamma it takes more than that. It takes a block of lead. Because as you know Alpha has a high mass, because it consists of the Helium element. That’s why it only takes a paper to stop it. Beta has a small particle. It’s not near as big as Alpha because it needs an iron plate to stop it. Because Beta sends out an electron or a positron. An electron is 2000 times smaller than a proton. Remember a proton and a positron is not the same! Gamma is energy, and that’s why it takes more than an iron plate to stop it. So why can lead stop it? As it is, Gamma belongs in the EM wave class. That means that EM waves get absorbed during the penetration of the lead. What absorbs the energy? It’s the electrons. They absorb the energy and converts it into heat or light. Because Gamma can generate heat with the help of the electrons from the lead, it can maybe in the future be used in many products. But the main source of this, will be to use it to make electricity. You could also combine positrons and a electrons in a controlled environment. That will force them together and make an explosion and form energy, used to make electricity.
Alpha-radiation is not very penetrating, so that means that a little simple paper can stop the radiation. There need to be 8 pieces of paper to stop Alpha-radiation.
Beta-radiation is a little bit more penetrating than Alpha, so Beta can’t be stop by paper. Aluminium can stop Beta-radiation. There need to be 8 pieces of paper and round about 2 mm aluminium.
Gamma-radiation is very penetrating, so it is hard to stop the energy. There need to be 7 cm lead before Gamma is slowed down.
Everyone on the earth, is exposed to radiation every hour. But it isn’t dangerous. Our organism is build for that, so it can’t hurt us. We can’t do anything to stop the daily radiation. This is called background radiation.
What do we use Alpha-radiation for?
Alpha is used in fire alarms or smoke detectors because in the fire alarm there is some Americium-241. There is like 1/5000 of a gram in it, but Americium sends out Alpha radiation. Americium constantly releases Alpha which knocks the electrons of the air. It ionizes the oxygen and nitrogen which are in the chamber. The positive oxygen and nitrogen are attached to the negative plate while the electrons are attached to the positive plate. That generates a small electric current. When smoke enters the chamber, the smoke particles attach to the ions and neutralise them. The effect of that is, that the ions don’t reach the plate. It stops the electric current and triggers the fire alarm.The Alpha particle is also in the atomic bomb. Uranium-235 sends out Alpha particle. The Alpha particle hits the other Uranium atoms and a nuclear fission is about to be created instantly. When a fission happens it generates a lot of heat, and then the big iron barrel can’t hold the pressure anymore.
What do we use Beta-radiation for?
We use Beta-radiation to cure people of cancer. When people get cancer in their cells, they go to a hospital. At the hospital they get radiotherapy. A machine, shoot radiation beams, at the place where you have got cancer. The radiation beams destroy the cells in which you have got cancer, and that will help you out of cancer. There are some side effects. When the machine shoot radiation beams at the place in which you have got cancer, you also loose the good cells. The worst that can happen is that you can loose your hair or you can get sterile. You can only be sterile if you get radiation beams in your testicles.
What do we use Gamma-radiation for?
Often when a fusion happens it creates energy in form of Gamma radiation. Gamma rays are the most energetic form of light and are produced in the very hot area of the universe. They are also produced in a supernova explosion. A supernova is a way to describe how massive stars die, like you have heard before, our sun is a star, not a planet. When the sun gets older and older, it grows to a massive size. By the end, the size of the sun will fill the whole solar-system and completely destroy all the planets. When the sun is at it’s biggest, it explodes. Not just a normal tiny explosion, but an explosion that is so powerful that it could erase the Milkyway. That is a supernova. Gamma rays are used in astronomy. Every star, supernova, black hole, solar flares and active galaxies send out gamma rays. When we invented the telescope which could see gamma rays, everything became much more clear. We could now experience a whole new level of exploring the galaxies.
Nuclear Phychics
Nuclear phychics were discovered in 1896 by a man called Henri Becquerel. In a coincidence he discovered that uranium salt blackened his photographic plates. He let the uranium salt be in his window for about 3 hours. The uranium salt was put on top of photographic plates. The uranium salt was wrapped in photographic film, so the sunshine couldn’t shine at the uranium salt. That is how he discovered it. In 1903 he got the Nobelprice for that discovery. Later they discovered that the reason of the blackened photographic plates was the changes in the unstable nuclear core.
This is also about radiation. If something emits Alpha radiation, it gives out 2 protons and 2 neutrons. Alpha is the largest radiation beam which exists. So it will go from one element to another.This is the solution:241Am237Np+4He. If you got 241Am and it releases alpha radiation, then that means 4helium molecule, travel away from it. Then it gets 4 less nucleons and then it becomes another element 237Np. But of course it takes time before an element becomes another element. That is what we call half-life. Half life is the time it takes to become another element. When a half life is gone 50% of it is covered into another element, now in this reason it’s Neptunium. When another half life is gone, 50% percentages of the rest of the Americium is covered into Neptunium. So now there’s only 25% left. This will happen until all of it, is covered into Neptunium. One half life of Americium is 432 years.
This doesn't happen in Beta radiation. In Beta radiation something else happen. Beta radiation sends out one proton and one electron. This happens when a neutron is transmuted into a proton and an electron. When a core gets another proton it becomes another element.
So Strontium sends out an electron and a proton. That will then say that the next element will get another proton. In this case it’s another Strontium molecule. When that happens it get another proton and therefore it will change into another element. The half life of Strontium is right around 29 years.
Gamma radiation is a electromagnetic beam. It is pure energy, and when an element emits gamma, none element transfusion happens. It doesn’t change into another element, it stays the same. Gamma radiation is in the same league as X-rays and Radio waves.
By Emil and Mikkel (9.A)
Labels:
English
Location:
Vonsild Skole, 6000 Kolding, Danmark
Weakening of radiation
PointThe point of these experiments is to see which radiation which have the longest range and highest intensity. It’s also an experiment which shows us how much resistance they can fly through.
Materials:
Alfa-, Beta-, and Gamma sources. Geiger tube and counter. Profile track with two riders. A ruler. Absorb set with different plates with different sizes. Other things like paper.
Experiment 1:
We started with the Alfa-source. We placed it in one of the riders and in the opposite rider we placed the Geiger tube, which was connected to a Geiger counter. We placed the Alfa-source close to the Geiger tube. Then we turned on the Geiger counter and heard really many beeps (which means that the intensity is high). Then we took the rider with the Alfa-source and moved it further from the Geiger tube. When the rider was moved a bit the beeps became less.
We removed the Alfa-source from the rider again and placed the Beta-source in the rider. We turned off the Geiger counter and moved the Beta-source rider close to the Geiger tube again. We turned on the Geiger counter again and heard many beeps but not as many as the Alfa-source. We moved the rider with the Beta-source further from the Geiger counter again and this time we noticed that there still were many beeps in a longer range than before.
We removed the Beta-source from the rider again and placed the Gamma-source. We turned off the Geiger counter and moved the Gamma-source close to the Geiger tube. Then we turned on the Geiger counter again and heard medium numbers of beeps. Then we started to move the Gamma-source further from the Geiger tube and this time we noticed and were very surprised that the beeps stopped between where the Alfa- and Beta-sources’ beeps stopped. Our teacher has told us that this experiment had been a failure. The Gamma-source was suppose to last much longer than the Beta-source. It was ought to sound like that the beeps still were intact when the Gamma-source was very far away.
Experiment 2:
We started with the Alfa-source and pointed it towards the Geiger tube. We put a paper between but it didn’t have any effect so we folded the paper once. It still didn’t have effect so we kept folding. The beam first got weakened when we had folded the paper three times and then had 8 layers of paper.
Then we tried with the Beta-source and we saw that paper didn’t had any effect at all. We tried therefore with some thicker aluminium-plates. The beam got weakened when 2 mm aluminium hang between the Beta-source and the Geiger tube. We saw a clear change. The Beta-source was obviously more persistent than the Alfa-source.
Then it became the Gamma-source’s turn. Here we had to adopt a tough stance. Neither paper or aluminium had any effect. The intensity first started dropping when we used lead. When the beam finally got weakened 1,5 cm of lead, 1 cm of aluminium and 32 layers of paper hang as brakes.
Theory:
If we have an arm, Alfa-radiation will stop on the skin, while Beta-radiation stops inside the arm and finally Gamma-radiation doesn’t stop. It flies right through the arm. These sources are radioactive.
The decay-process for the 3 radiations:
The actual Alfa-radiation is (we can take Radon as an example) when Radon transmits a Helium-core (consisting of two protons and two neutrons) because of it’s unstability. When Radon has transmitted the Helium-core (Alfa-particle), it becomes the element named Polonium.
Beta-radiation often starts because the nucleus is unstable (it has too many neutrons). Therefore the nucleus emit an electron (a Beta-particle) and now the nucleus transforms a neutron to a proton. The nucleus is positive and because it has got a proton more, it changes into the element just above it in the periodic table (if it was number 55 before, then it is number 56 now. It had got and extra proton and the numbers of protons correspond to the element’s number in the periodic table).
Caesium gains a proton and transforms into Barium while it emits an electron.
In our daily life radioactive radiation is nothing we should be afraid of. We have in fact a radioactive isotope of Kalium in our body. Background radiation is mostly coming from the sky or the earth and isn’t dangerous either. It’s in tiny amounts so we are not affected by it, even though it hits us every day. If a power station explodes, thousands of radioactive matters flies out in our atmosphere and can be carcinogenic or deadly.
Konklusion:
We found out that Alfa-radiation had a short range but a very high intensity close up. Beta-radiation had a not quite as intense radiation but it had a longer range than Alfa-radiation. Gamma-radiation had the longest range. It was not that intense but it was almost unstoppable. Gamma is pure energy/electromagnetic radiation so it doesn’t react like the others. The Alfa-particle is the biggest (It consists of a Helium-core (two protons and two neutrons and the core is positive)) it weighs 4 unit. The Beta-particle is the smallest. It consists of an electron and is therefore negative. It weighs 1/2000 of a unit.
We proved that the Alfa-particle is the biggest given that we could stop it with something as thin as paper. We proved that the Beta-particle was smaller given that it could fly through much more robust materials.
By Frederik og Christian 9.A
Labels:
English
Location:
Vonsild Skole, 6000 Kolding, Danmark
Subscribe to:
Comments (Atom)



